Lead Carbonate Nitric Acid Reaction . Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. Pbo + hno3 = pb(no3)2 + h2o is a double displacement (metathesis) reaction where one mole of lead(ii) oxide [pbo] and two moles of. Nitric acid + lead carbonate = lead(ii) nitrate + water + carbon dioxide. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. Nitric acid, and lead (ii) oxide. P bo(s) + 2h n o3(aq) → p b((n o)3)2(aq) + h 2o(l) answer link. Lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Two moles of nitric acid [hno 3] and one mole of lead carbonate [pbco. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,.
from www.youtube.com
Lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Two moles of nitric acid [hno 3] and one mole of lead carbonate [pbco. Pbo + hno3 = pb(no3)2 + h2o is a double displacement (metathesis) reaction where one mole of lead(ii) oxide [pbo] and two moles of. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. Nitric acid, and lead (ii) oxide. P bo(s) + 2h n o3(aq) → p b((n o)3)2(aq) + h 2o(l) answer link. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected. Nitric acid + lead carbonate = lead(ii) nitrate + water + carbon dioxide. Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water.
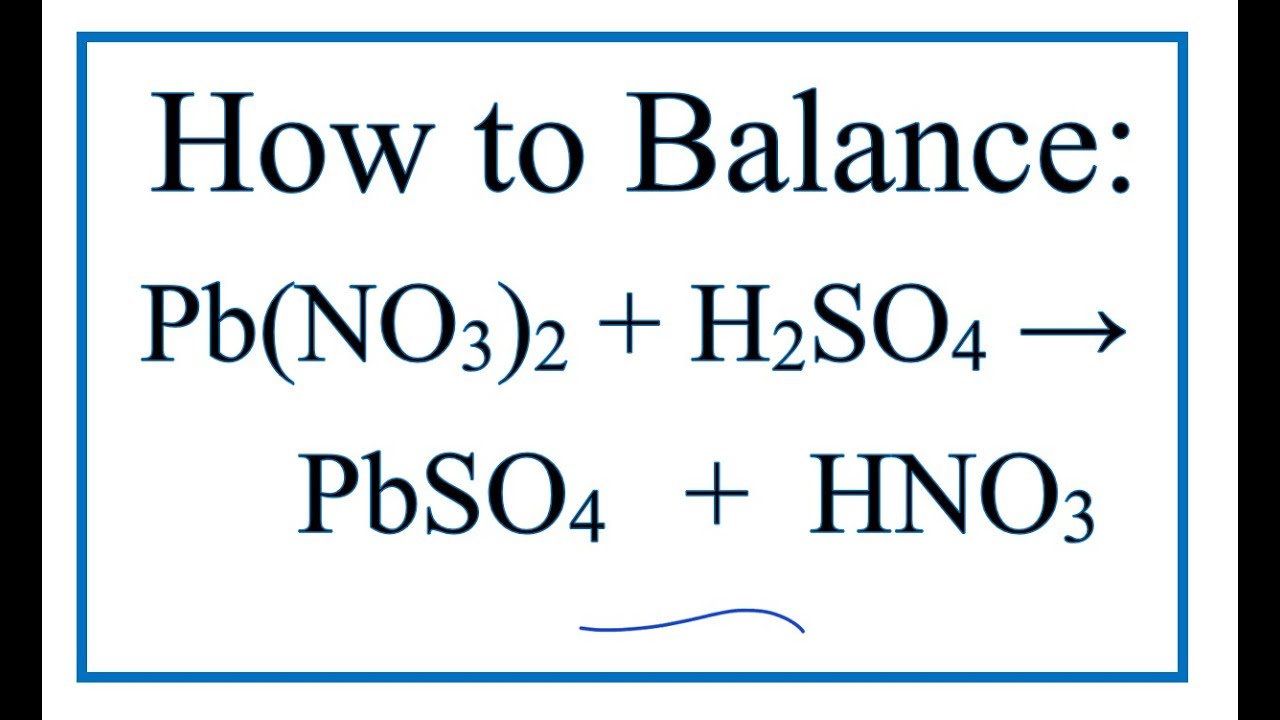
How to Balance Pb(NO3)2 + H2SO4 = PbSO4 + HNO3 Lead (II) nitrate
Lead Carbonate Nitric Acid Reaction Two moles of nitric acid [hno 3] and one mole of lead carbonate [pbco. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. Nitric acid, and lead (ii) oxide. Lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Two moles of nitric acid [hno 3] and one mole of lead carbonate [pbco. P bo(s) + 2h n o3(aq) → p b((n o)3)2(aq) + h 2o(l) answer link. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected. Nitric acid + lead carbonate = lead(ii) nitrate + water + carbon dioxide. Pbo + hno3 = pb(no3)2 + h2o is a double displacement (metathesis) reaction where one mole of lead(ii) oxide [pbo] and two moles of. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,.
From www.chegg.com
Solved Write a balanced equation for the reaction of nitric Lead Carbonate Nitric Acid Reaction When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,. Nitric acid, and lead (ii) oxide. Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. Nitric acid + lead carbonate = lead(ii) nitrate + water + carbon dioxide. Lead(ii) ion reacts with aqueous ammonia to. Lead Carbonate Nitric Acid Reaction.
From www.chegg.com
Solved Predict Products, And Write Balanced Net Ionic Equ... Lead Carbonate Nitric Acid Reaction Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected. Pbo + hno3 = pb(no3)2 + h2o is a double displacement (metathesis) reaction where one mole of lead(ii) oxide [pbo] and two moles of. Nitric acid, and lead (ii) oxide. Nitric acid will react with a metal carbonate or bicarbonate to form a. Lead Carbonate Nitric Acid Reaction.
From www.youtube.com
Equation for Mg(NO3)2 + H2O (Magnesium nitrate + Water) YouTube Lead Carbonate Nitric Acid Reaction Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. P bo(s) + 2h n o3(aq) → p b((n o)3)2(aq) + h 2o(l) answer link. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,. We were trying to dissolve a piece of metallic lead in. Lead Carbonate Nitric Acid Reaction.
From www.coursehero.com
[Solved] Does magnesium nitrate and sulfuric acid react together? If so Lead Carbonate Nitric Acid Reaction Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. Lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. Pbo + hno3 = pb(no3)2. Lead Carbonate Nitric Acid Reaction.
From www.youtube.com
How does Nitric acid reacts with metals YouTube Lead Carbonate Nitric Acid Reaction Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. Nitric acid, and lead (ii) oxide. Pbo + hno3 = pb(no3)2 + h2o is a double displacement (metathesis) reaction where one mole of lead(ii) oxide [pbo] and two moles of. Nitric acid + lead carbonate = lead(ii) nitrate + water + carbon dioxide.. Lead Carbonate Nitric Acid Reaction.
From www.youtube.com
Reaction of Nitric Acid with Carbonates and Bicarbonates, Chemistry Lead Carbonate Nitric Acid Reaction Nitric acid, and lead (ii) oxide. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. Lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. Lead Carbonate Nitric Acid Reaction.
From www.pinterest.com
Test for a Carbonate infographic diagram showing a laboratory Lead Carbonate Nitric Acid Reaction Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,. Lead. Lead Carbonate Nitric Acid Reaction.
From www.teachoo.com
Case Based Class 10 Science The physical states of the reactants Lead Carbonate Nitric Acid Reaction Two moles of nitric acid [hno 3] and one mole of lead carbonate [pbco. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected. Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. Pbo + hno3 = pb(no3)2 + h2o is a double displacement (metathesis). Lead Carbonate Nitric Acid Reaction.
From oxygengasnaraeru.blogspot.com
Oxygen Gas April 2017 Lead Carbonate Nitric Acid Reaction Nitric acid, and lead (ii) oxide. P bo(s) + 2h n o3(aq) → p b((n o)3)2(aq) + h 2o(l) answer link. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected. Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. We were trying to dissolve. Lead Carbonate Nitric Acid Reaction.
From www.youtube.com
Lead carbonate with Nitric acid YouTube Lead Carbonate Nitric Acid Reaction Nitric acid, and lead (ii) oxide. Nitric acid + lead carbonate = lead(ii) nitrate + water + carbon dioxide. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,. Lead when reacted with nitric acid forms. Lead Carbonate Nitric Acid Reaction.
From brainly.com
Solid lead(11) carbonate reacts with a dilute solution of nitric acid Lead Carbonate Nitric Acid Reaction Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected. Two moles of nitric acid [hno 3] and one mole of lead carbonate [pbco. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. When acids react with. Lead Carbonate Nitric Acid Reaction.
From www.youtube.com
When zinc reacts with very dilute nitric acid it produces YouTube Lead Carbonate Nitric Acid Reaction Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected. P bo(s) + 2h n o3(aq) → p b((n o)3)2(aq) + h 2o(l) answer link. Lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until. Lead Carbonate Nitric Acid Reaction.
From www.showme.com
Ashok (sodium carbonate and nitric acid) total ionic equation Science Lead Carbonate Nitric Acid Reaction Lead when reacted with nitric acid forms lead(ii) nitrate, nitrogen oxide and water. Pbo + hno3 = pb(no3)2 + h2o is a double displacement (metathesis) reaction where one mole of lead(ii) oxide [pbo] and two moles of. Two moles of nitric acid [hno 3] and one mole of lead carbonate [pbco. When acids react with carbonates, such as calcium carbonate. Lead Carbonate Nitric Acid Reaction.
From www.numerade.com
Lead (II) carbonate to form lead (II) oxide and carbon Lead Carbonate Nitric Acid Reaction Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. Nitric acid + lead carbonate = lead(ii) nitrate + water + carbon dioxide. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,. Nitric acid, and lead (ii) oxide. Lead when reacted with nitric acid forms. Lead Carbonate Nitric Acid Reaction.
From pubs.acs.org
Reactions of Metals in Nitric Acid Writing Equations and Calculating Lead Carbonate Nitric Acid Reaction Nitric acid, and lead (ii) oxide. Nitric acid + lead carbonate = lead(ii) nitrate + water + carbon dioxide. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,. Pbo + hno3 = pb(no3)2 + h2o is a double displacement (metathesis) reaction where one mole of lead(ii) oxide [pbo] and two moles of.. Lead Carbonate Nitric Acid Reaction.
From mungfali.com
Nitration Of Phenol Mechanism Lead Carbonate Nitric Acid Reaction Pbo + hno3 = pb(no3)2 + h2o is a double displacement (metathesis) reaction where one mole of lead(ii) oxide [pbo] and two moles of. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,. Two moles of nitric acid [hno 3] and one mole of lead carbonate [pbco. Nitric acid, and lead (ii). Lead Carbonate Nitric Acid Reaction.
From www.youtube.com
Write the balanced chemical equation of the following word equation Lead Carbonate Nitric Acid Reaction We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. Two moles of nitric acid [hno 3] and one mole of lead carbonate [pbco. P bo(s) + 2h n o3(aq) → p b((n o)3)2(aq) + h 2o(l) answer link. Pbo + hno3 = pb(no3)2 +. Lead Carbonate Nitric Acid Reaction.
From alendrasung.blogspot.com
Function Of Nitric Acid Nitric acid molecule Stock Image F004 Lead Carbonate Nitric Acid Reaction Nitric acid will react with a metal carbonate or bicarbonate to form a salt, co2 and water. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected. We were trying to dissolve a piece of metallic lead in concentrated nitric acid until we found out that concentrated nitric acid is not a. Pbo. Lead Carbonate Nitric Acid Reaction.